Abstract
Background: It has recently become apparent that the immune system can cure cancer. There are variety of methods for generating these immune responses. In some of these strategies, such as CAR-T cells and the somatic mutation vaccines, the antigen targets are pre-identified and therapies are custom-made against these targets for each patient. In other approaches, such as checkpoint antibodies, "off the shelf" antibodies are used to remove the breaks on the immune system, allowing pre-existing T cells to attack unknown targets on cancer cells.
We have developed another non-customized approach called in situ vaccination. Here, immune enhancing agents are injected locally into one site of tumor, that triggers a T cell immune response locally that then travels to attack cancer throughout the body.
In order to screen for the best local immune stimulatory agents, we have employed a preclinical strategy whereby the same syngeneic tumor is implanted at two separate sites in the body. One tumor is then injected with the test agents and the resulting immune response is detected by the regression of the distant, untreated tumor.
Using this assay for abscopal therapeutic effects we have screened a variety of ligands for Toll-Like Receptors (TLRs), antibodies against immune checkpoints and T cell co-stimulatory targets in multiple combinations of these agents.
Among these, the combination of unmethylated CG-enriched oligodeoxynucleotide (CpG) -a TLR9 ligand and anti-OX40 antibody has provided the most impressive results. TLRs are components of the innate immune system that recognize molecular patterns on bacterial, fungal, or viral pathogens. Intratumoral (IT) injection of CpG, results in tumor eradication at the injected site but no in systemic anti-tumor immune response. However, it induced the enhanced expression of OX40 on the local tumor-infiltrating T cells. The addition of antibody against OX40, a potent costimulatory activator of T cells, resulted in eradication of both local and distant disease.
This treatment resulted in a T cell immune response that could be measured both in vitro and in vivo. The systemic anti-tumor response required the presence of both CD4+ and CD8+ T cells as mice treated with the corresponding depleting antibodies were unable to control tumor growth.
In addition, the cured animals were protected from re-challenge with the same A20 tumor but not unrelated tumors.
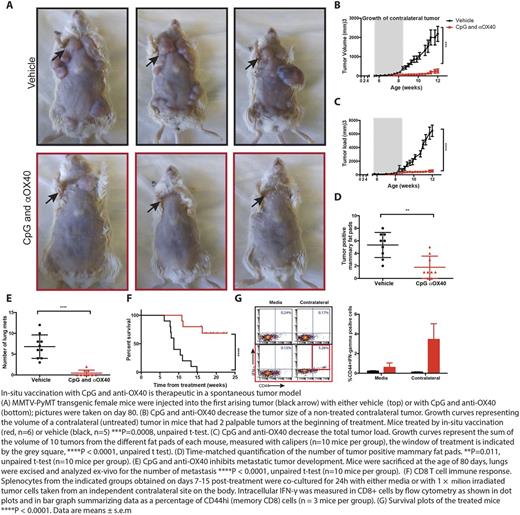
To examine the potential of in situ vaccination to treat spontaneous cancers, we chose MMTV-PyVT a mouse tumor model that allowed for local injected at a predicable site of disease. These animals develop invasive breast cancer in all of their mammary glands by 12 weeks of age and widespread lethal metastases by 20 weeks of age. Injections of CpG and anti-OX40 antibody into the first arising tumor not only prevented its growth but prevented the outgrowth of subsequent tumors at un-injected mammary glands, reduced lung metastases and significantly improved overall survival.
Impact: Anti-OX40 and CpG are both currently in phase-I trials as single agents. Each has proved safe with slight evidence of anti tumor effects. Our results provide strong rationale for using these two agents in combination as an in situ therapeutic vaccine for lymphoma tumors.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.